Welcome to Fides Biocare
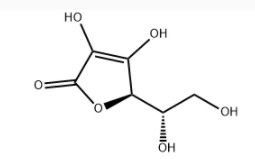
FIDES BIOCARE.are Manufacturer, Supplier and Exporter of variegated Pharma products that consists of Finished Products, OTC products, Nutraceutical Medicines. • We are evolved as a trailblazer in Pharmaceutical industry, operated office at Pharma hubSurat in (Gujarat), India. We envisage being provider of quality products across the globe in Human &VeterinaryPharmaceutical products. • We promise better healthcare and longer life for every human being at very affordable prices. Fides Group Inc.is dedicated to development, manufacturing and marketing of quality Pharmaceutical to meet growing demand of healthcare. • We cater to the demands of the Asia Pacific, Latin America, Middle-East, Africa, CIS country, Asia Pacific etc. by understanding their requirements and accordingly developing pharmaceutical drugs as we do under contractual manufacturing. • We have the flexibility to meet your needs under strict quality guidelines. • We approach our business seriously and provide value and quality to our customers every day. Fides Bioexport devotes extensive effort in providing medicines to third world countries. Tablets Lotion Oral Liquid Eye / Ear Drops Dry Powder Toothgel syrup Mouthwash Capsules Irrigation solutions Softgel Capsules Small volume parenterals Injectables Large volume parentrals Ointments Reconstituted powders Cream Anti-infective Hormonals Antituberculosis Anti-Asthmatics/Cough and Cold and Nasal Sprays Oncological Products Gaestro-intestinal Products Antimalarial cardiovascular/Lipid lowering Agents Psychotropic and Antidepressants Bone modulating Products Analgesic Urological and Erectile dysfunction Products Dermatological Products Heamatonics and Nutritional supplements Anti-diabetic Gynecological Products Appetite Stimulant Veterinary medicines Diabetic and Endocrine Products other • Preparation of Dossier files As per country specific guidelines • Review of dossier file As per the Regulatory Authority’s guidelines • Assistance in MOH schedule for product life cycle management • Filing of Registration dossiers for submission to various regulatory agencies all over the world (including CTD format & ACTD Format). • Non-clinical and clinical overviews and summary writing through literature search from published studies / registered studies and research articles having good impact factor. • Drafting the MRA query during the registration procedures and responding to the same • Post approval Changes or reregistration for product life cycle management by assisting the clients for additional data generation •
-
FOUNDER and CEO
DHAARMI PATEL
-
Year of Establishment
2009
-
Primary Business
Retailer
-
Number of Employees
21 - 50
-
Annual Turnover
Below Rs. 0.5 Crore Approx.