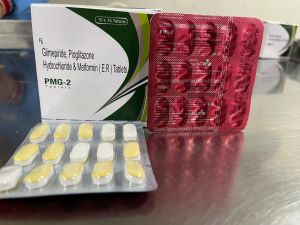
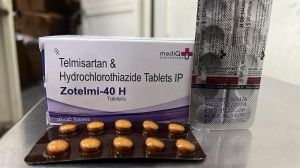
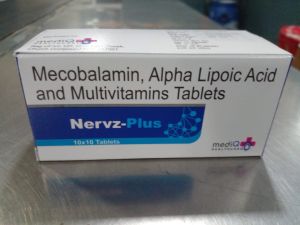
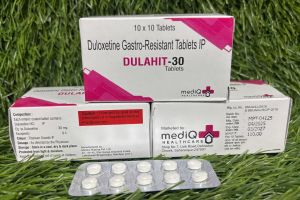
Welcome to Mediq Healthcare
Quality and Regulatory Compliance 1. *Good Manufacturing Practices (GMP)*: Ensuring compliance with GMP standards to guarantee product quality and safety. 2. *Regulatory Approvals*: Obtaining necessary approvals from regulatory bodies, such as the FDA or EMA. ## Production Process 1. *Formulation and Development*: Creating the medicine's formula and developing the manufacturing process. 2. *Raw Material Sourcing*: Procuring high-quality raw materials from reliable suppliers. 3. *Manufacturing*: Producing the medicine according to the approved formula and process. ## Time Limit and Rate The time limit and rate for manufacturing medicine can vary greatly depending on factors such as: 1. *Product Complexity*: The type of medicine being manufactured, its formulation, and production process. 2. *Production Capacity*: The manufacturer's production capacity, equipment, and resources. 3. *Regulatory Requirements*: Compliance with regulatory standards and approvals. To provide a more accurate estimate, it's essential to know the specific details of the medicine being manufactured, such as: 1. *Type of medicine*: What type of medicine is being manufactured (e.g., tablets, injectables, liquids)? 2. *Quantity*: What is the required quantity of the medicine? 3. *Production timeline*: What is the desired production timeline? With more information, manufacturers can provide a more accurate quote and timeline for production.
-
Sales manager
Junaid ali
-
Year of Establishment
2019
-
Primary Business
Manufacturer
-
Number of Employees
6 - 20
-
Annual Turnover
Rs. 5 to 25 Crore Approx.