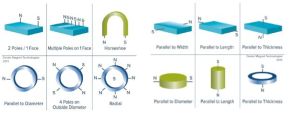
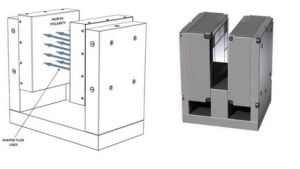
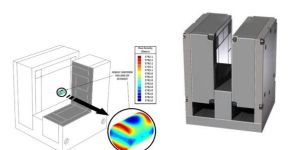
Magnetic Resonance Imaging (MRI) was known as Nuclear Magnetic Resonance (NMR) until the ‘nuclear’ connotation became unpopular, but both names denote the Magnetic Resonance (MR) principle involved. Besides the MRI machines one finds in hospitals, magnetic resonance devices are commonly in devices used to ensure proper chemistry material mix, such as equipment used to monitor quality of asphalt. MR detects an atom’s gyromagnetic ratio, the ratio of the magnetic dipole moment, due to nuclear spin, to the mechanical angular momentum, to discriminate between elements. Almost every element in the periodic table has an isotope with a non zero nuclear spin, but to be useful the isotope must also be abundant in the volume being analyzed. Therefore, the nuclei of interest in MRI of the human body and other living organisms are those of hydrogen, carbon, nitrogen, sodium, phosphorus, potassium and calcium.MRI system components include: a magnetic dipole to establish a static magnetic field, a gradient coil, an RF coil to produce an alternating magnetic field at 90° to the static magnetic field, and an antenna coil. In operation, protons in the sample volume are oriented by the static field, and caused to precess by the alternating RF magnetic field. When power to the RF coil is turned off, the magnetic moment of the protons realigns with the static magnetic field. The energy change involved in realignment of the magnetic moments is measured as a small RF signal by the antenna coil and a Fourier transform of signal frequency and phase produces data used to construct a distinctive image.