Company Information
Ask for more detail from the seller
Contact SupplierAg/AgCl Reference Electrode
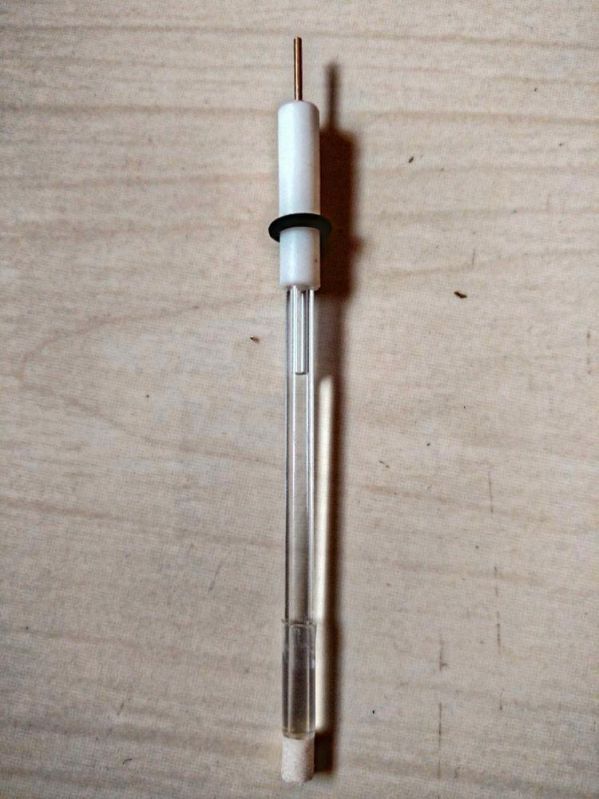
An Ag/AgCl reference electrode is one of the most commonly used reference electrodes in electrochemical measurements. It consists of a silver wire coated with silver chloride, immersed in a solution containing a known concentration of chloride ions (commonly KCl). This design provides a stable and well-defined electrode potential, which is independent of the test solution, making it reliable for precise electrochemical experiments.
Key Features:
Construction: Silver wire coated with AgCl, placed in a chloride ion-containing electrolyte.
Stability: Offers a stable and reproducible potential over long periods.
Compatibility: Non-toxic compared to mercury-based electrodes; suitable for aqueous electrochemical systems.
Applications: Used widely in corrosion studies, cyclic voltammetry, battery and fuel cell research, biosensors, and general electrochemical testing.
Advantages:
Simple design and easy handling
High reproducibility of potential
Environmentally safer than calomel electrodes