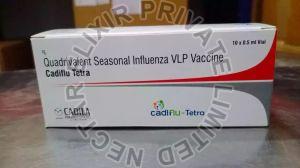
Listing ID #1224801
Company Information
Ask for more detail from the seller
Contact SupplierFLUARIX, Influenza Vaccine, for intramuscular injection, is a sterile colorless and slightly opalescent suspension. FLUARIX is a vaccine prepared from influenza viruses propagated in embryonated chicken eggs. Each of the influenza viruses is produced and purified separately. After harvesting the virus-containing fluids, each influenza virus is concentrated and purified by zonal centrifugation using a linear sucrose density gradient solution containing detergent to disrupt the viruses. Following dilution, the vaccine is further purified by diafiltration. Each influenza virus solution is inactivated by the consecutive effects of sodium deoxycholate and formaldehyde leading to the production of a “split virus.” Each split inactivated virus is then suspended in sodium phosphate-buffered isotonic sodium chloride solution. The vaccine is formulated from the 3 split inactivated virus solutions.
FLUARIX has been standardized according to USPHS requirements for the 2014-2015 influenza season and is formulated to contain 45 micrograms (mcg) hemagglutinin (HA) per 0.5-mL dose, in the recommended ratio of 15 mcg HA of each of the following 3 strains: A/Christchurch/16/2010 NIB-74XP (H1N1) (an A/California/7/2009-like virus), A/Texas/50/2012 NYMC X-223A (H3N2), and B/Massachusetts/2/2012 NYMC BX-51B.
FLUARIX is formulated without preservatives. FLUARIX does not contain thimerosal. Each 0.5-mL dose also contains octoxynol-10 (TRITON® X-100) ≤ 0.085 mg, a-tocopheryl hydrogen succinate ≤ 0.1 mg, and polysorbate 80 (Tween 80) ≤ 0.415 mg. Each dose may also contain residual amounts of hydrocortisone ≤ 0.0016 mcg, gentamicin sulfate ≤ 0.15 mcg, ovalbumin ≤ 0.05 mcg, formaldehyde ≤ 5 mcg, and sodium deoxycholate ≤ 50 mcg from the manufacturing process.
The tip caps and plungers of the prefilled syringes of FLUARIX are not made with natural rubber latex.