




Company Information
Ask for more detail from the seller
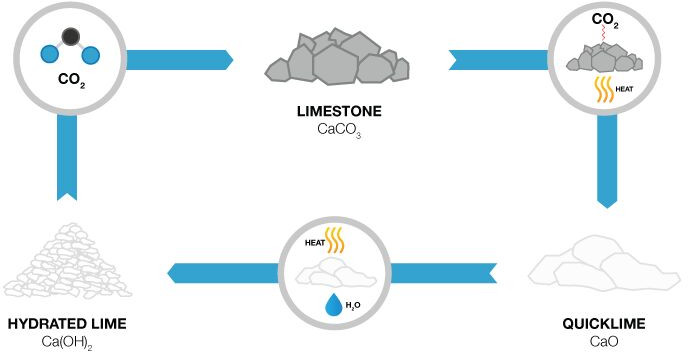
Contact SupplierHydrated lime, Calcium hydroxide (Ca(OH)2), also called slaked lime, is a white free flowing dry powder produced commercially when Quick Lime (CaO) is treated with proportionate amount of water under the chemical reaction : CaO + H2O → Ca(OH)2.
We process Hydrated Lime of purity from 70% to 92% having our manufacturing base both at KATNI (M.P.) & MANAKPUR (RAJASTHAN). Our Hydrated Lime is processed by picking the best 100% Calcined Limestone from best of the mines of area i.e. Quick Lime with addition (spraying) of proportionate quantity of water (preferably hot) yielding least moistured free flowing dry powder, thereafter packed into quality HDPE Bags.
APPLICATIONS :
Construction: Hydrated lime is used to stabilize soils for roads, dams, airfields, and building foundations. It can also be used to raise the pH of acidic soils, a process known as "soil sweetening"
Water and wastewater treatment: Hydrated lime is used for acid neutralization, water softening, and impurity removal. It can also be used to treat flue gas emissions.
Steel manufacturing: The use of Hydrated Lime is in the steel industry, where hydrated lime neutralizes impurities in plants where coke is a by-product. Further, It's soluble spray is also used in Continuous Casting Machine & Pig Iron to keep out hot yield of pig iron sticking with each other.
In Ferro Alloy industries it is used as Binder with Ore for Briquett formation.
Chemical manufacturing: Hydrated lime is used in a variety of chemical manufacturing applications like Bleaching Powder, Di Calcium Phosphate,basic component like bromide, caustic soda, fluoride, magnesia, lactate, nitrate, oleate, and stearate. Many organic and inorganic calcium salts, such as calcium carbide, calcium hypochlorite, calcium magnesium acetate, and calcium phosphate, begin production with hydrated lime. Hydrated lime also plays a role in citric acid purification.
Flue gas treatment: Hydrated lime catalyzes particles emanated after combustion in cement plants, coal fire plants, glass industries, and other incendiary plants. Acidic pollutants normally released into the environment, such as hydrogen fluoride, hydrogen chloride, sulfur dioxide, selenium, and fine particulate matter are captured by hydrated lime’s anions, eventually rendering them into calcium sulfate.
Sugar production: Both cane and beet sugar rely on hydrated lime to react with impurities and elevate pH. Hydrated lime also removes impurities in the manufacture of maple syrup, sorghum, or other viscous forms of sugar. Carbonation before the final product is packaged removes excess lime.
Masonry binding: Hydrated lime is used to bind with sand to form plaster and stucco. Adding 15% hydrated lime to cement significantly reduces shrinkage and cracking. Road and building foundations, earthen dams, and airfields all rely on hydrated lime.
Refractory : Hydrated Lime of high purity is used in making valuable refractory product "High Alumina Cement"
Leather tanning: Hydrated lime is used in the tanning of leather.
Food Industries : When the plant material is processed with water to form a juice, the juice's pH is acidic and replete with impurities. Adding hydrated lime adjusts the pH and flocculates the impurities for removal.
There are so many more usages of Hydrated Lime.
Please feel free to send any query about Hydrated Lime.






