




Company Information
Ask for more detail from the seller
Contact SupplierNiksan Pharmaceutical is leading manufacturer and distributer of theEdaravoneamong the other countries. Niksan Pharmaceutical is one of the major the supplier, distributer, exporter and manufacturer of the EdaravoneAPI and finished formulations in Ankleshwar, Gujarat, India. The products of Niksan Pharmaceutical and Niksan group companies are widely appreciated by the clients and other companies.
Niksan Pharmaceutical is the supplier, manufacturer, exporter and trader of Edaravonein the domestic level as well as the international market.
Niksan Pharmaceutical provides EdaravoneAPI in all over Indian states like Jammu & Kashmir, Himachal Pradesh, Uttarakhand, Punjab, Kerala, Chandigarh, Chhattisgarh, Andhra Pradesh, Rajasthan, Meghalaya, Mizoram, Nagaland, Orissa, Pondicherry, Dadra and Nagar Haveli, Goa, Gujarat, Jharkhand, West Bengal, Karnataka, Kerala, Madhya Pradesh etc.
Niksan Pharmaceutical also provides EdaravoneAPI and finished formulations in all other countries of the world like Germany, Switzerland, Poland, Latvia, Egypt, Lithuania, Estonia, Ukraine, Moldova, Russia, United States, Austria, Belarus, Serbia, India, Spain, Slovenia, Nigeria, Croatia, South Africa, Kazakhstan, Italy, Romania, Bulgaria, Portugal, South Korea, Greece, Philippines, Belgium, Saudi Arabia, Turkey, Japan etc.
Edaravoneis one type of medication . It may work to slow the nerve damage associated with the worsening of ALS symptoms.
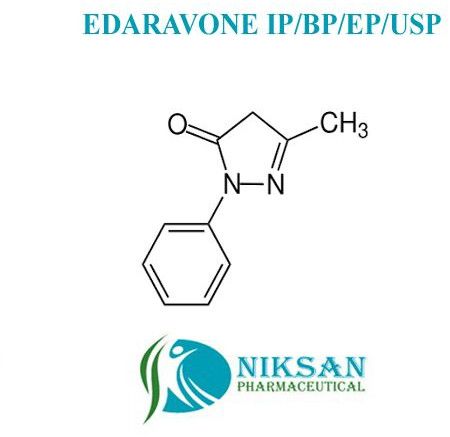
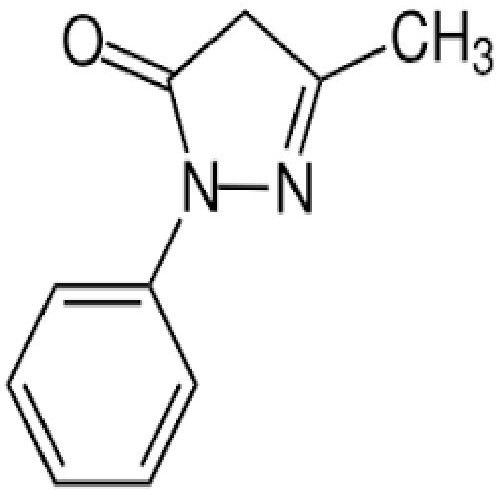
SYNONYMS: Edaravone, Methylphenylpyrazolone, Norphenazone, Phenyl methyl, pyrazolone, Phenylmethylpyrazolone
IUPAC NAME:5-methyl-2-phenyl-4H-pyrazol-3-one
CAS NO: 89-25-8
FORMULA: C10H10N2O
MOLECULAR MASS: 174.203 g/mol
STORAGE OF EDARAVONE:Store at up to 25°C (77°F). Excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from light. Store in overwrapped package to protect from oxygen degradation until time of use.
APPLICATIONS OF EDARAVONE:Edaravone injection is used to treat amyotrophic lateral sclerosis (ALS, Lou Gehrig's disease; a condition in which the nerves that control muscle movement slowly die, causing the muscles to shrink and weaken). Edaravone injection is in a class of medications called antioxidants.
HOW TO USE: The recommended dose of edaravone is 60 mg administered via 60-minute IV infusion once daily for 14 days as the initial treatment cycle, followed by a 14-day drug-free period. Subsequent treatment cycles consist of once-daily dosing for 10 of 14 days, each followed by a 14-day drug-free period.
HOW EDARAVONE WORKS: The recommended dose of edaravone is 60 mg administered via 60-minute IV infusion once daily for 14 days as the initial treatment cycle, followed by a 14-day drug-free period. Subsequent treatment cycles consist of once-daily dosing for 10 of 14 days, each followed by a 14-day drug-free period.
CONTRAINDICATIONS OF EDARAVONE: Edaravone is contraindicated for use in patients with a history of a hypersensitivity to edaravone or any of the inactive ingredients, including sulfite hypersensitivity. Hypersensitivity reactions, including anaphylactic reactions, have occurred with edaravone.
PHARMACOKINETICS OF EDARAVONE: Edaravone is metabolized to a sulfate conjugate and a glucuronide conjugate, which are not pharmacologically active. The glucuronide conjugation of edaravone involves multiple uridine diphosphate glucuronosyltransferase (UGT) isoforms (UGT1A1, UGT1A6, UGT1A7, UGT1A8, UGT1A9, UGT1A10, UGT2B7, and UGT2B17).
SIDE EFFECTS OF EDARAVONE: In some cases, Blistering, crusting, irritation, itching, or reddening of the skin, blue lips, fingernails, or skin, chest pain or tightness, change in walking and balance, clumsiness or unsteadiness,cough, Confusion, racked, dry, scaly skin.
Note: Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk







