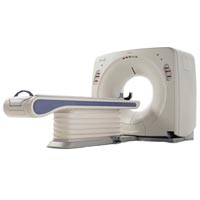
Listing ID #1119681
Company Information
Ask for more detail from the seller
Contact SupplierThe company is also a competent provider of Healthcare Regulatory Services for clients across the globe. The Healthcare Regulatory Services made available by the company can be availed at the best prices in the market, with proper quality assurance methods. The Healthcare Regulatory Services that we provide are handled by the most experienced and well-trained professionals. We also make sure that all the requirements are met, with the provision of our Healthcare Regulatory Services.
Scope of Work