

Company Information
Ask for more detail from the seller
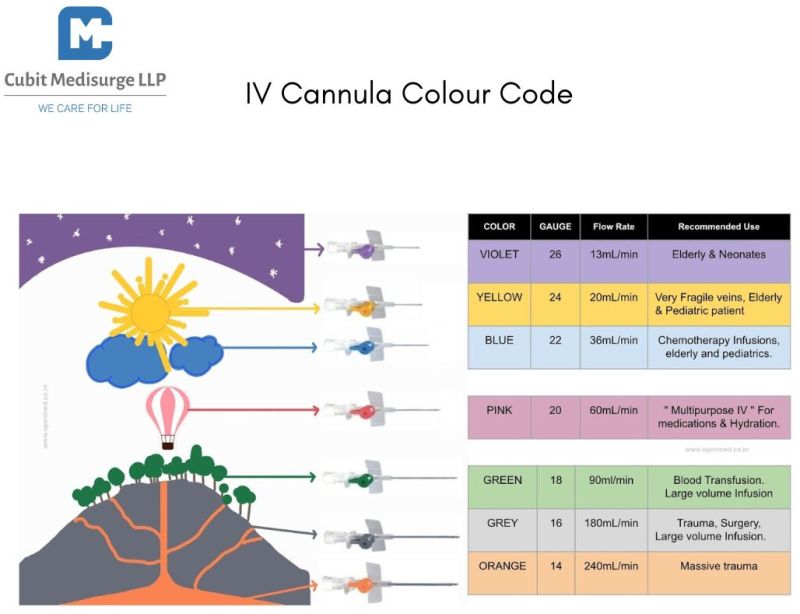
Contact SupplierThe 26G IV Cannula is a sterile, single-use medical device designed for peripheral intravenous access, especially in neonates, infants, pediatric patients, and elderly individuals with fragile or small veins. It enables safe and effective administration of fluids, medications, or blood products directly into the bloodstream. Its fine gauge and ultra-sharp needle ensure painless and atraumatic insertion.
🔧 Key Features:
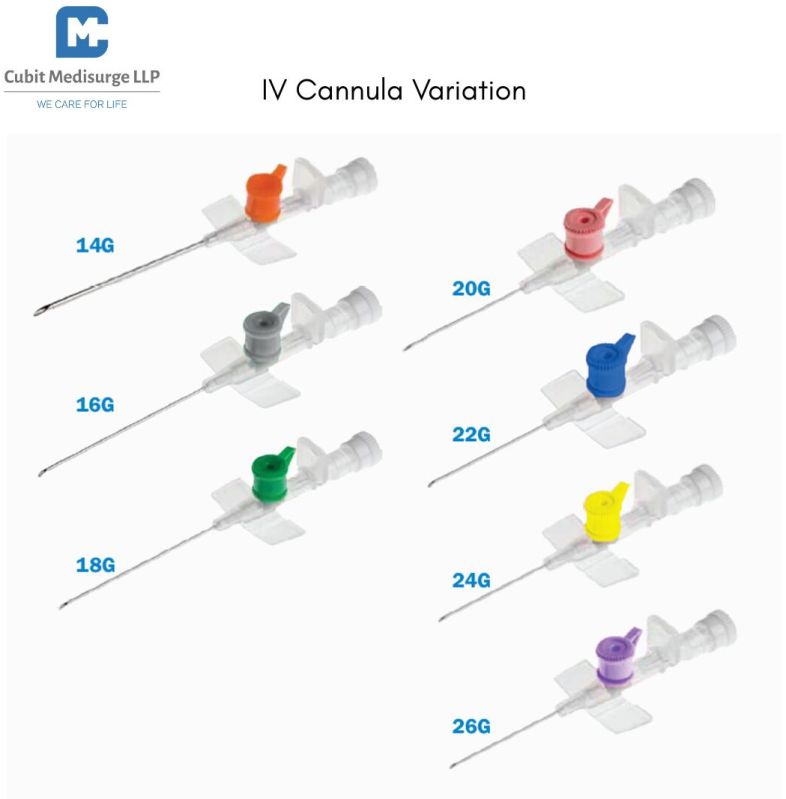
Size: 26G – Ultra-fine for delicate veins
Color-coded hub (Purple) as per international standards for easy identification
Triple-facet, beveled needle tip for smooth and painless vein entry
Integrated injection port (optional) for intermittent drug delivery without needle
Flashback chamber for immediate visualization of venous access
Flexible and kink-resistant catheter made from medical-grade PTFE or polyurethane
Hydrophobic filter in flashback chamber to prevent blood spillage
Universal luer lock cap for secure closure when not in use
Sterile, non-toxic, pyrogen-free, latex-free, and DEHP-free construction
📐 Technical Specifications:
| Gauge Size | 26G |
| Outer Diameter (OD) | Approx. 0.6 mm |
| Catheter Length | 19–24 mm (varies by manufacturer) |
| Flow Rate | Approx. 13 mL/hr |
| Catheter Material | PTFE / Polyurethane |
| Needle Material | Stainless steel (SS 304 or 316) |
| Sterilization | EO (Ethylene Oxide) |
| Packaging | Individually packed in sterile pouch |
| Shelf Life | 3–5 years |
✅ Applications:
Neonatal and pediatric IV access
Elderly patients with fragile veins
Use in NICUs, PICUs, pediatric wards, and emergency care
Low-volume fluid and medication administration
📦 Available Variants:
With or without injection port
With wings or wingless (for stabilization)
Safety/Needle-Protected IV Cannula (optional)
Radio-opaque catheter (for imaging)
🧾 Certifications (if applicable):
CE Mark
ISO 13485




