Company Information
Ask for more detail from the seller
Contact SupplierChemical Name
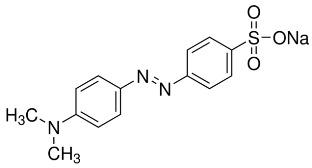
p-((p-(Dimethylamino)phenyl)azo)benzenesulfonic acid sodium salt
InChI Key
STZCRXQWRGQSJD-UHFFFAOYSA-M
Solubility
In water= 200 mg/l at 25 deg C. 20 mg/ml in 2-methoxyethanol; 0.3 mg/ml in ethanol
Methyl orange does not have a full spectrum of color change, but has a sharper end point. Methyl orange shows red color in acidic medium (pH < 3.1) and yellow color in basic medium (pH > 4.4)
Applications:
Methyl orange is also used in dyeing and printing textiles as a dyestuff. Also used as an indicator of acid-base titration, and the alkalinity of the water. Also for textile dyeing. Methyl orange is a pH indicator frequently used in titrations. Because it changes colour at the pH of a mid-strength acid, it is usually used in titrations for acids. Indicators are weak acids or bases which change color depending on the pH of the solution. Because they have such vivid color changes, only small amounts of indicators must be added to a solution which also limits any side effects they might have on the reaction being observed. Methyl orange is a pH indicator frequently used in titrations. Methyl orange is also used for histological microscopy.At pH values less than 3.1, methyl orange is red and a pH values greater than 4.4, it will be yellow. In the range between 3.1 and 4.4, you will see a mixture of the red and yellow colors such that in the middle of this range, the solution will appear to be orange. Since acidic solutions have low pH values, it will be red and transition to orange and yellow as the acidity of the solution decreases. In basic solutions, it will be yellow as well as in neutral solutions because the pH of a neutral solution is approximately 7 and falls after the color change of methyl orange.

